Rupture of Membranes Tests Market to Reach USD 1,892.9Mn 2036 Milestone as Hospital Protocol Adoption Sustains Demand
Hospital protocols and maternal care guidelines are driving steady global adoption of rupture of membranes tests across maternity units.
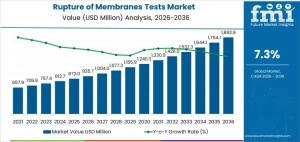
NEWARK, DE, UNITED STATES, January 19, 2026 /EINPresswire.com/ -- The rupture of membranes tests market is entering a period of sustained expansion, driven by standardized hospital protocols, rising procedural volumes in maternity care, and the need for rapid, accurate diagnostics during labor. Global spending on ROM tests is projected to reach USD 935.7 million by 2026 and is forecast to grow to USD 1,892.9 million by 2036, reflecting a compound annual growth rate (CAGR) of 7.3%, according to the latest market assessment updated on 15 January 2026.
This growth is occurring primarily within hospital maternity units, obstetrics centers, and perinatal networks across Asia Pacific, North America, and Europe. Demand is shaped less by brand recognition or promotional activity and more by clinical validation, workflow compatibility, and adherence to maternal care guidelines. Once a test is validated for routine use, hospitals require parallel testing and quality verification before approving substitutions, reinforcing long-term supplier relationships.
Market Context: Why Rupture of Membranes Testing Matters
Rupture of membranes tests are used to confirm amniotic fluid leakage during suspected preterm or term labor. Early and accurate detection plays a critical role in guiding clinical interventions, reducing infection risk, and supporting appropriate antibiotic use. Traditional approaches such as visual inspection or nitrazine testing are associated with higher false-positive rates, increasing the risk of unnecessary interventions.
Modern ROM tests rely on biochemical markers, immunoassays, or fluorescence-based detection, delivering objective results with faster turnaround times. Hospitals and birthing centers evaluate these tests based on sensitivity, specificity, shelf life, storage requirements, and ease of sample collection, as well as their ability to integrate into electronic health records and reporting systems.
Growth Forecast Through 2036
In 2026, ROM test demand is concentrated in maternity wards and high-risk obstetric units where testing is embedded into standardized care pathways. Purchasing decisions are tied to the number of equipped labor and delivery units rather than short-term fluctuations in birth rates. As hospitals expand capacity for high-risk pregnancies and formalize testing protocols, uptake continues to rise.
By 2036, as market value approaches USD 1.9 billion, supply chain readiness and procedural efficiency become more influential. Buyers increasingly prioritize reagent stability, batch consistency, ease of use, and predictable delivery across multi-site hospital networks. Growth reflects cumulative adoption across maternal health systems rather than a single regulatory change or replacement of existing diagnostic methods.
Key Market Metrics
• Market Value (2026): USD 935.7 million
• Forecast Value (2036): USD 1,892.9 million
• Forecast CAGR (2026–2036): 7.3%
• Leading Test Type: Immunoassay-based tests
• Key Growth Regions: Asia Pacific, North America, Europe
Product Type and Clinical Integration
Immunoassay-based tests account for approximately 56% of market demand due to their high sensitivity and suitability for point-of-care use in maternity wards. These systems require consistent calibration, regulatory documentation, and ongoing quality checks, positioning suppliers as long-term partners rather than transactional vendors.
Rapid strip tests support bedside decision-making with minimal operator training, while multiplex tests address specialized requirements in tertiary centers where multiple biomarkers are assessed simultaneously. Once a hospital standardizes on a product type, switching costs remain high due to revalidation, staff training, and quality assurance requirements.
Application Trends Driving Volume
Preterm premature rupture of membranes (preterm PROM) diagnosis represents around 66% of total demand. Early detection in these cases is critical to prevent neonatal complications and guide timely interventions. Term PROM testing, while lower in volume, remains essential to reduce infection risk and unnecessary procedures, supporting stable demand across maternity care settings.
Regional Demand Outlook
Country-level growth reflects differences in healthcare infrastructure and maternity service expansion:
• India: 11.0% CAGR, driven by organized maternity services and expanding maternal health programs
• China: 10.8% CAGR, supported by large hospital networks and standardized maternal care initiatives
• Brazil: 10.3% CAGR, reflecting growth in private hospital networks
• United States: 9.8% CAGR, shaped by routine protocol-driven use in obstetrics departments
• Germany: 8.0% CAGR, indicating a mature, guideline-driven market
Competitive Landscape
Competition in the ROM tests market centers on assay reliability, regulatory compliance, and workflow integration. Leading players including Qiagen, Hologic, Abbott, Roche, and Artron compete at the clinical evaluation stage, where sensitivity, specificity, turnaround time, and documentation are assessed. Once approved, validation files, quality control processes, and training programs anchor long-term adoption.
Market positions vary by hospital type and regulatory environment, with molecular assay expertise, analyzer compatibility, platform integration, and operational simplicity influencing supplier selection.
Explore trends before investing – request a sample report today! https://www.futuremarketinsights.com/reports/sample/rep-gb-31378
Outlook
The rupture of membranes tests market is increasingly defined by clinical accuracy, standardized protocols, and recurring demand tied to patient throughput rather than short-term purchasing cycles. As maternal health systems continue to formalize diagnostic pathways, ROM tests are positioned as essential tools in modern obstetric care.
Browse Related Insights
Stress Tests Equipment Market: https://www.futuremarketinsights.com/reports/stress-tests-equipment-market
Hematocrit Tests Market: https://www.futuremarketinsights.com/reports/hematocrit-tests-market
Dental Repair Membranes for Implant Procedures Market: https://www.futuremarketinsights.com/reports/dental-repair-membranes-for-implant-procedures-market
Sudip Saha
Future Market Insights Inc.
+18455795705 ext.
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.