Breast Cancer Subtyping Kits Market Forecast 2026-2036: Market Size, Share, Competitive Landscape
Precision Oncology Gains Momentum: Global Breast Cancer Subtyping Kits Market Projected to Reach USD 12.0 Billion by 2036
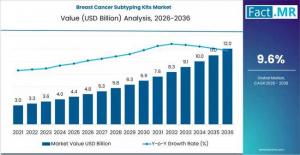
ROCKVILLE, MD, UNITED STATES, January 21, 2026 /EINPresswire.com/ -- A comprehensive market study by Fact.MR reveals that the global Breast Cancer Subtyping Kits Market is entering a transformative era of growth. Valued at USD 4.8 billion in 2026, the market is forecasted to expand at a steady CAGR of 9.6%, reaching a valuation of USD 12.0 billion by the end of 2036.As oncology shifts toward personalized treatment, subtyping kits have emerged as indispensable tools for clinicians. These kits allow for the precise identification of molecular markers—such as HER2 status, Hormone Receptor (HR) expression, and Triple-Negative profiles—enabling healthcare providers to move away from one-size-fits-all therapies and toward targeted, more effective patient care.
Request for Sample Report | Customize Report |purchase Full Report - https://www.factmr.com/connectus/sample?flag=S&rep_id=13750
Answering the Critical Needs of Modern Oncology
The surge in demand for breast cancer subtyping kits is driven by several pivotal factors:
Who: Leading healthcare players, including Roche Diagnostics, Agilent Technologies, Abbott Laboratories, and Thermo Fisher Scientific, are spearheading innovation in the sector.
What: Advanced diagnostic kits—specifically IHC-based kits and PCR-based assays—are being deployed to categorize tumors with high accuracy.
Where: While North America and Europe currently maintain significant market shares, the Asia-Pacific region, led by India (11.1% CAGR) and China (10.8% CAGR), is emerging as the fastest-growing frontier due to rising healthcare infrastructure and cancer awareness.
Why: Early and accurate subtyping is directly linked to higher survival rates. The rising global incidence of breast cancer, coupled with the emergence of targeted therapies like CDK4/6 and PARP inhibitors, necessitates more granular diagnostic data.
How: Market growth is being facilitated by favorable regulatory frameworks (such as ISO 13485 and CE marking) and the integration of AI-enhanced digital pathology to reduce human error.
IHC-Based Kits and Hospitals Lead Segment Growth
According to the report, IHC-based (Immunohistochemistry) kits remain the dominant product type, accounting for 46.0% of the market share. This dominance is attributed to their cost-effectiveness and deep integration into established clinical workflows.
Simultaneously, hospitals continue to be the primary end users, commanding a 48.0% share of the market. The concentration of specialized oncologists, pathologists, and integrated patient care models within hospital settings makes them the central hub for advanced molecular testing and diagnosis.
Strategic Market Context and Regional Outlook
The report underscores a significant shift in the diagnostic landscape:
North America: The U.S. market is projected to grow at a 9.2% CAGR through 2036, driven by a robust precision medicine sector and high adoption rates of novel molecular diagnostics.
Emerging Markets: Brazil and India are witnessing double-digit growth rates, supported by government-subsidized screening programs and an expanding middle class with better access to specialty healthcare.
Innovation: The industry is seeing a rise in Liquid Biopsy technology and PCR-based circular supply chain models, which offer non-invasive alternatives for real-time cancer monitoring.
Future Outlook: The Role of Personalized Medicine
By 2036, the integration of AI-powered machine learning and automated histopathology is expected to further compress diagnostic timelines. As healthcare systems globally transition from reactive to proactive models, breast cancer subtyping kits will play a foundational role in the burgeoning USD 49.5 billion breast cancer therapeutics market.
The transition from traditional pathology to molecular-driven diagnostics is no longer a luxury but a clinical necessity, states a Fact.MR analyst. The subtyping kits market is the engine driving the next generation of survival-focused oncology.
About Fact.MR Fact.MR is a leading provider of market intelligence and consulting services, serving 80% of Fortune 1,000 companies. With a global presence and a portfolio of over 1,000 reports annually, Fact.MR delivers data-backed insights that empower industry leaders to make informed strategic decisions.
To View Related Report :
Antibiotic Residue Test Kits Market
https://www.factmr.com/report/496/antibiotic-residue-test-kits-market
Timing Belt Kits Market
https://www.factmr.com/report/709/timing-belt-kits-market
Pacemaker/Defibrillator Lead Extraction Kits Market
https://www.factmr.com/report/858/pacemaker-defibrillator-lead-extraction-kits-market
Animal Pregnancy Test Kits Market
https://www.factmr.com/report/935/animal-pregnancy-test-kits-market
About Fact.MR:
We are a trusted research partner of 80% of fortune 1000 companies across the globe. We are consistently growing in the field of market research with more than 1000 reports published every year. The dedicated team of 400-plus analysts and consultants is committed to achieving the utmost level of our client’s satisfaction.
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.